Page History
Background
The University of Sydney will be is undertaking an Atrial Fibrillation (AF) study within ten General Practices. The study involves clinicians utilizing an existing mobile device to ascertain if the patients pulse indicates to be normal or patient's pulse indicates a normal result reading or otherwise otherwise. The mobile device will be used as a decision support tool. The AF Topbar app will notify the clinician when patients are eligible for the mobile device.
The Atrial Fibrillation AF app will only display a notification, based on the following requirements:
- Patient is 65 years old and over
- Patient does not have a coded diagnosis of Atrial Fibrillation
- Patient has no history of having the mobile device test completed within the last 12 months
When a patient does not meet the qualifying criteria, the following message will present:
- Patient does not meet qualifying c riteria
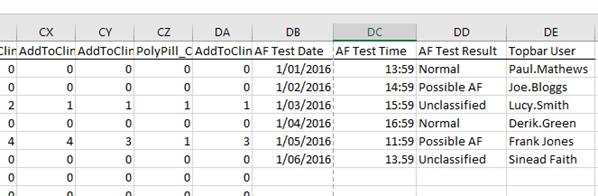
The data collected through the AF app in Topbar will be extracted extracted by CAT4 and added to the file sent to 'The George Institute'. The four additional fields added to the file are:
- AF Test Data (dd/mm/yyyy)
- AF Test Time
- AF Test Result
- Topbar username
Only patient patients that have data stored in the Topbar database will be able to populate the cells in the CSV. If a patient does have the appropriate data, then the cells will be blank.
Displaying the AF app
The AF app is displayed automatically when an eligibel eligible patient is opened in the clinical system .
...
The AF app can be turned off by clicking on the close option, or hidden using the 'hide' option.
...
and shows a small red circle with the number 1 to indicate one new notification.
...
Atrial Fibrillation App use
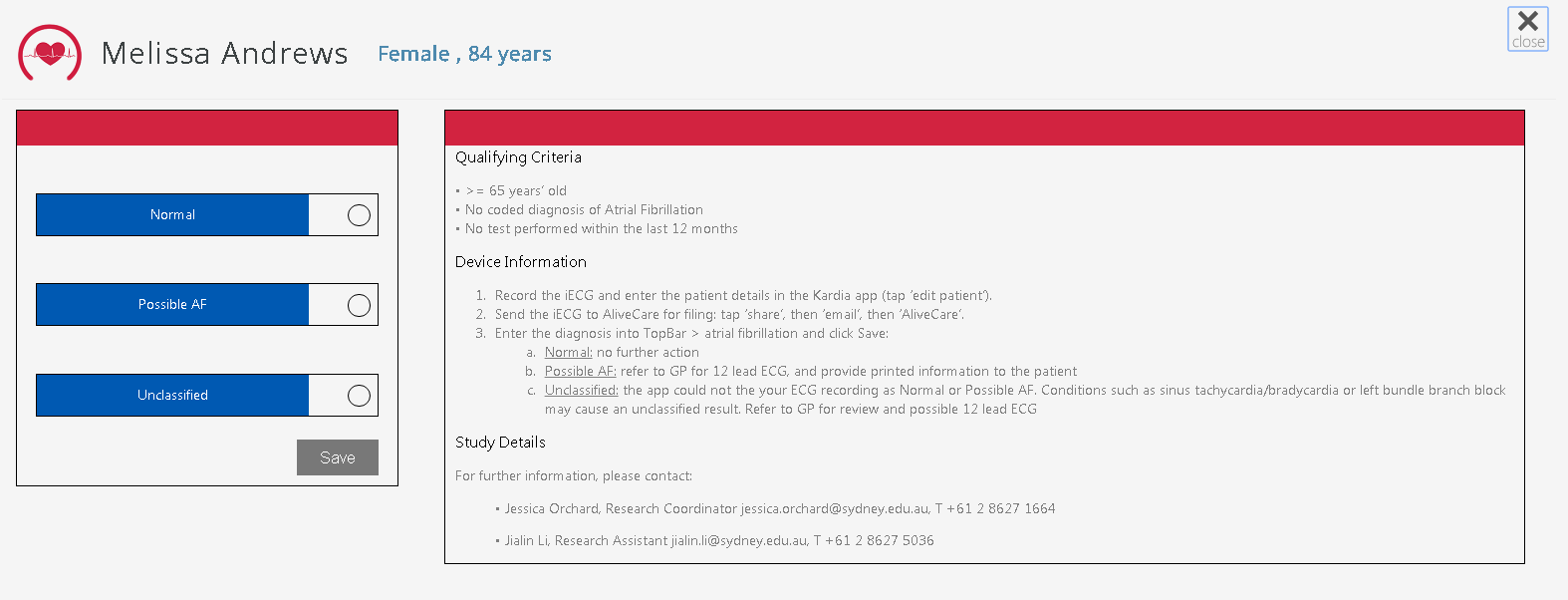
Clicking on the tile for the AF app will open the full screen view for the currently opened patient:
...
The screenshot above is missing the 'Device Information' and the 'Study Details' text. In the final version the text displayed will be:
The following text will be displayed regardless of whether a patient meets the qualifying criteria or not.
Device Information
|
Study Details For further information, please contact: Jessica Orchard, Research Coordinator jessica.orchard@sydney.edu.au, T +61 2 8627 1664 Jialin Li, Research Assistant jialin.li@sydney.edu.au, T +61 2 8627 5036 |
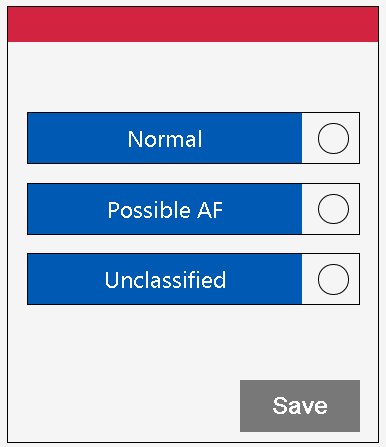
When a user wants to record the patients pulse result, delivered and reported through the mobile device, the three following radio button options are available for selection;
...
A user can only select one at any given point. By default, all options will appear de-selected and the 'Save' button is greyed out.
Only once a selection has been made the 'Save' button will become active.
...
The Test Result will be visible to the user during the first 12 months from the date of the Test Result being saved.
Once an outcome of the test (Normal, Possible AF or Unclassified) is recorded, there are no further actions in the AF app. The app has now disappeared from Topbar and will not be shown again for this patient for the next 12 months and then only if the patient still meets the eligibility criteria described in the introduction. It will appear again when the next eligible patient is opened in the clinical system.