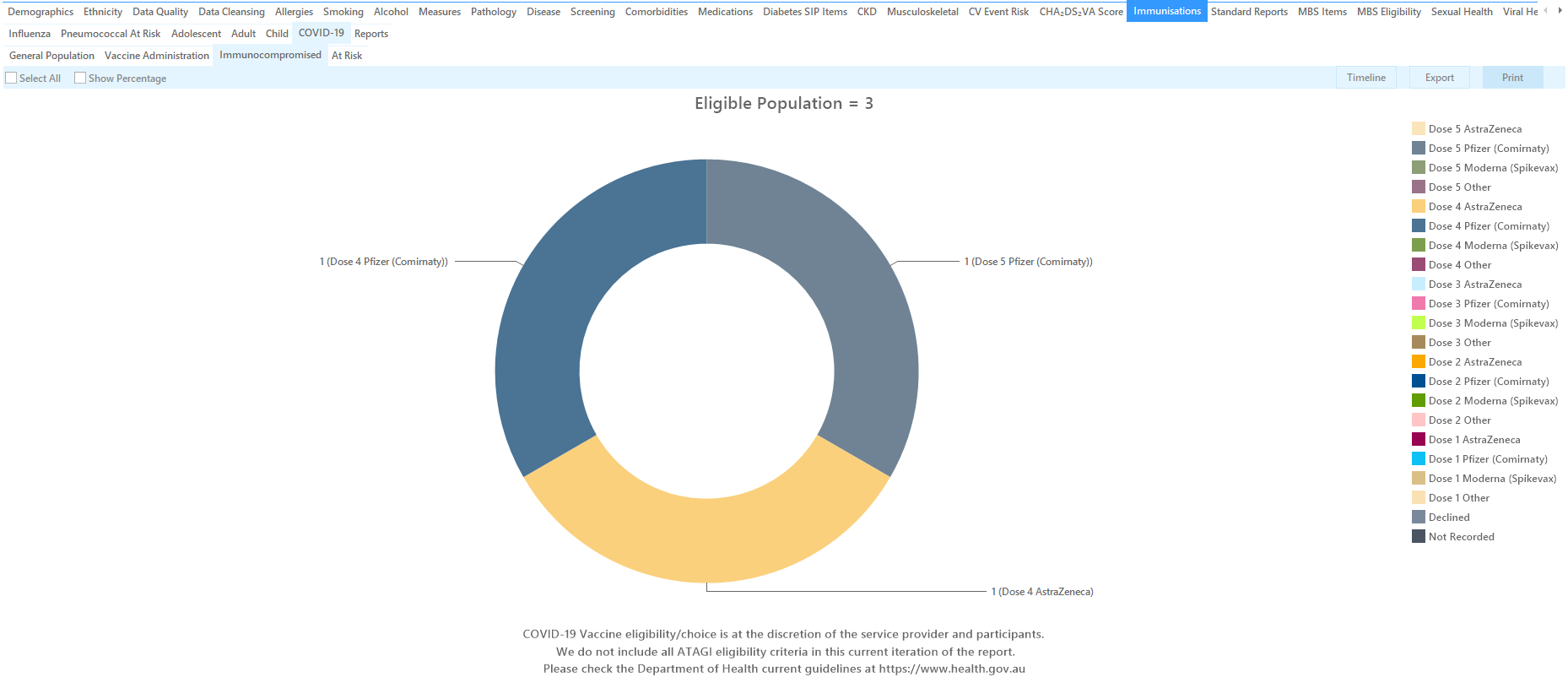
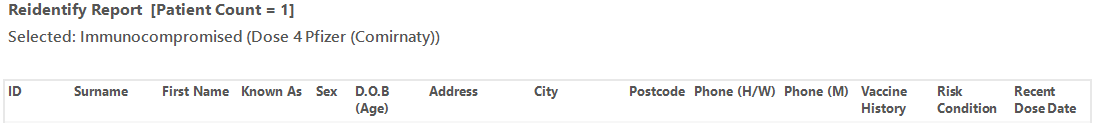
This report shows patients with immunocompromising conditions and there vaccine status. It is based on the ATAGI advice that recommends the following:
ATAGI recommends a 3rd primary dose of COVID-19 vaccine in severely immunocompromised populations to address the risk of suboptimal or non-response to the standard 2 dose schedule.
The 3rd dose is intended to maximise the level of immune response to as close as possible to the general population. ATAGI recommends that all individuals aged ≥12 years with certain conditions or on therapies leading to severe immunocompromise, receive a 3rd primary dose of a COVID-19 vaccine.
The recommended interval for the 3rd dose is 2 to 6 months after the 2nd dose of vaccine. A minimum interval of 4 weeks may be considered in exceptional circumstances (e.g., anticipated intensification of immunosuppression; outbreaks).
People who have received a 2nd dose more than 6 months ago should receive a 3rd dose as soon as feasible.
In addition to the 3 primary doses, ATAGI also advises booster doses for immunocompromised patients represented as Dose 4 and Dose 5 in this report.
The report allows to filter by date range using the date range filter, and also lists the different vaccines and dose numbers.
The re-identification report shows the date of the last vaccine and the type of vaccine.
For this report we are using the ATAGI recommendations as detailed in this document: atagi-recommendations-on-the-use-of-a-third-primary-dose.pdf
| ATAGI Condition | Pen CS mapping approach |
|---|---|
| Active haematological malignancy | mapped for all active haematological cancers |
| Non-haematological malignancy with current active treatment including chemotherapy, radiotherapy, and/or hormonal therapy, but excluding immunotherapy with immune checkpoint inhibitors | mapped based on conditions and treatment as recorded in the patient record. |
| Solid organ transplant with immunosuppressive therapy | Mapped based on transplant recorded and medication recorded |
| Haematopoietic stem cell transplant (HSCT) recipients or chimeric antigen receptor T-cell (CAR-T) therapy within 2 years of transplantation. These patients require revaccination with 3 additional doses of COVID-19 vaccine, irrespective of doses given prior to transplantation, commencing generally ≥3-6 months after their transplant after discussion with their treating specialist. Those beyond 2 years from transplant should discuss with their treating specialist about the need for a 3rd dose. | Not mapped as too specific and due to time restraints. These patients should be known to the providers and require additional attention. |
| Immunosuppressive therapies | based on medication mappings as recorded |
| Biologic and targeted therapies anticipated to reduce the immune response to COVID-19 vaccine | As there was no definition provided, we are using all immunosuppressive medications as recorded |
| Primary immunodeficiency including combined immunodeficiency and syndromes, major antibody deficiency (e.g., common variable immune deficiency (CVID) or agammaglobulinemia), defects of innate immunity (including phagocytic cells), defects of immune regulation, complement deficiencies and phenocopies of primary immunodeficiencies. | Using general immunodeficiency conditions as recorded in the patient history |
| Advanced or untreated HIV with CD4 counts <250/μL or those with a higher CD4 count unable to be established on effective anti-retroviral therapy | Any diagnosis of HIV due to lack of options to identify 'advanced' or 'untreated' HIV |
| Long term haemodialysis or peritoneal dialysis | any dialysis due to lack of definition of 'long term' |