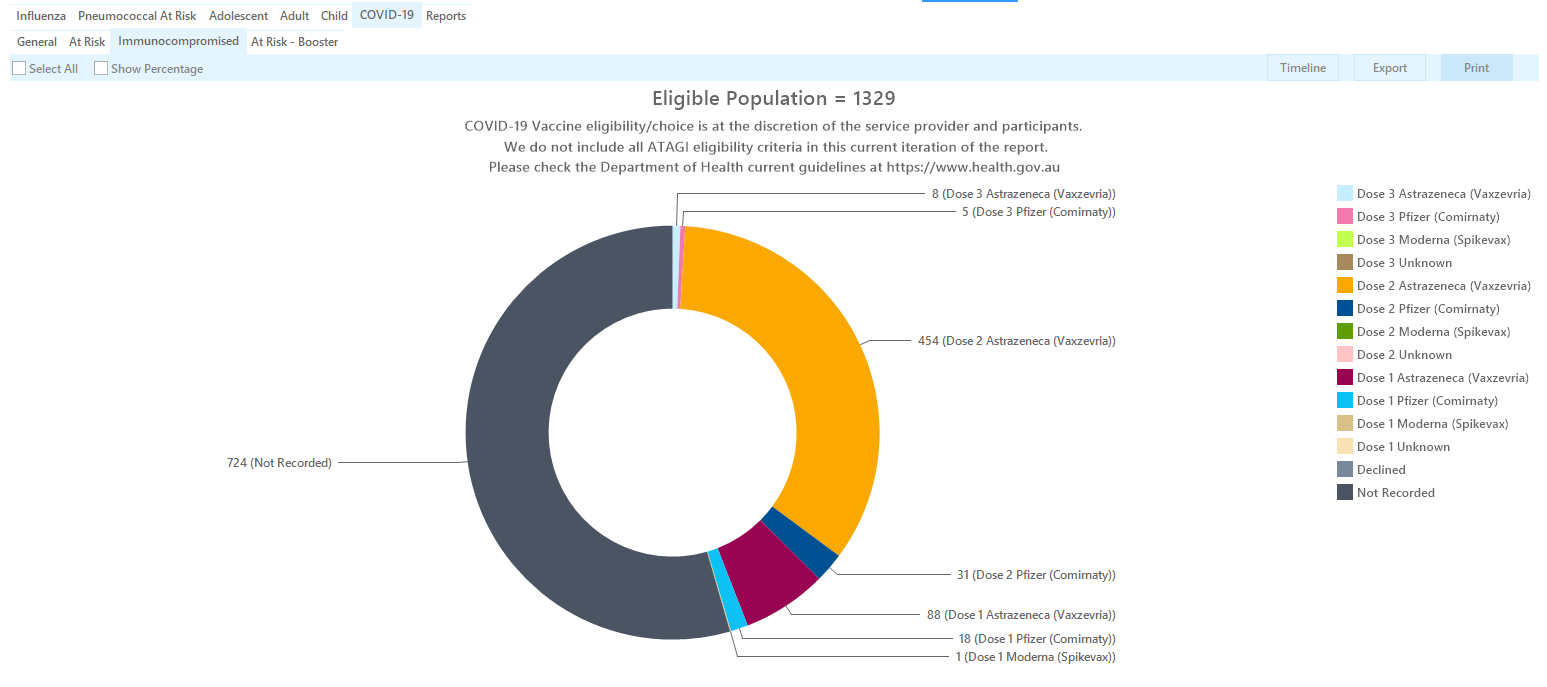
This report shows patients with immunocompromising conditions and there vaccine status. It is based on the ATAGI advice that recommends the following:
ATAGI recommends a 3rd primary dose of COVID-19 vaccine in severely immunocompromised populations to address the risk of suboptimal or non-response to the standard 2 dose schedule.
The 3rd dose is intended to maximise the level of immune response to as close as possible to the general population. ATAGI recommends that all individuals aged ≥12 years with certain conditions or on therapies leading to severe immunocompromise, receive a 3rd primary dose of a COVID-19 vaccine.
The recommended interval for the 3rd dose is 2 to 6 months after the 2nd dose of vaccine. A minimum interval of 4 weeks may be considered in exceptional circumstances (e.g., anticipated intensification of immunosuppression; outbreaks).
People who have received a 2nd dose more than 6 months ago should receive a 3rd dose as soon as feasible.
The report allows to filter by date range using the date range filter, and also lists the different vaccines and dose numbers.
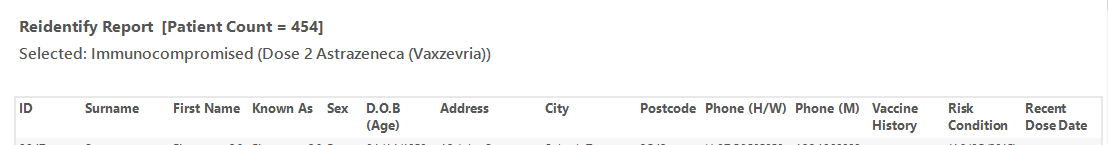
The re-identification report shows the date of the last vaccine and the type of vaccine.